ADVERTISEMENTS:
Natural Resources Term Paper – This is one of the best term papers on ‘Natural Resources’ especially written for school and college students.
Term Paper on Natural Resources
Term Paper Contents:
- Term Paper on the Introduction to Natural Resources
- Term Paper on the Classification of Natural Resources
- Term Paper on Air
- Term Paper on Water: A Precious Resource
- Term Paper on Soil
1. Term Paper on the Introduction to Natural Resources:
ADVERTISEMENTS:
Earth is the only planet on which life exits. The existence of life mainly depends on factors like, food,water,air,soil and energy from the sun. Some of these are natural while others are man – made like food.The common term used ids resources which are anything we can use from nature and environment to achieve our objective.
Natural resources are useful raw materials that we get from the earth. They occur naturally, which means that humans cannot produce natural resources. Instead, we use and modify them to make them beneficial to us. These are directly or indirectly responsible for human survival.
Natural resources are land, water and air, animals, minerals, sunlight, etc.
2. Term Paper on the Classification of Natural Resources:
There are different ways to classify natural resources, including where they come from and whether they are renewable or non-renewable.
ADVERTISEMENTS:
If natural resources come from living things or organic materials, then they are considered biotic resources. Biotic resources include plants, animals, and fossil fuels. The three fossil fuels are coal, oil, and natural gas. Fossil fuels are classified as biotic resources because they were formed from the decay of organic matter over millions of years.
On the other hand, abiotic resources originate from non-living and inorganic materials. For example, air, sunlight, and water are abiotic natural resources. Minerals (gold, copper, iron, and diamonds) are also considered abiotic.
ADVERTISEMENTS:
On the basis of their availability and abundance, they are of following two types:
i. Inexhaustible:
These cannot be exhausted by man’s consumption because of their excess amounts, e.g., air, sunlight, wind, etc. These are affected by over-population of mankind.
ii. Exhaustible:
These are limited in their quantity and can get exhausted over a period of time i.e. coal, petroleum, etc.
(a) Renewable Resources:
Renewable resources are those that can be replenished or reproduced easily. Some of them like wind, air, sunlight, etc. are continuously available. Their quantity is not affected by man’s consumption.
Some renewable resources can be depleted by human use but is also replenished, thus maintaining a flow. Agricultural crops take a short time for renewal. Water takes a comparatively longer time for renewal, while forest takes even longer time.
(b) Non-Renewable Resources:
Non-renewable resources are those which are formed over very long geological periods. Minerals and fossil fuels are included in this category. As the rate of formation of fossil fuels is extremely slow, they cannot be replenished once they get depleted. The metallic minerals can be recycled.
3. Term Paper on Air:
Air is inexhaustible resource made of a mixture of many gases. The composition of the air is maintained by the life forms present on earth. On planets like Mars and Venus, the atmosphere is mostly composed of carbon dioxide (95-97%).
Air is the mixture of many gases like oxygen, nitrogen, carbon dioxide, water vapor and other gases that are present in trace amount. Air as whole is required for survival of organisms as they breathe air. Hence, the name given of breathe of life.
When energy is required, eukaryotic and prokaryotic organism breakdown glucose molecules in the presence of oxygen which produces energy and carbon dioxide. Combustion is another process where oxygen is consumed and carbon dioxide is released when burning fuel.
Despite this, the percentage of carbon dioxide in our atmosphere is a mere fraction of a percent because carbon dioxide is ‘fixed’ in two ways:
Carbon Dioxide Fixation:
(i) Green plants convert carbon dioxide into glucose in the presence of sunlight and release oxygen in air.
(ii) Many marine animals use carbonates dissolved in sea-water to make their shells.
Role of Air in Climate Control:
The atmosphere acts as a blanket due to its following functions:
1. Temperature control: it maintains the average temperature of the earth fairly constant during the course of the year.
2. It prevents a sudden increase in temperature during daylight hours.
3. During the night, it slows down the escape of heat into outer space.
The moon is at an equal distance from earth and sun and has no atmosphere. Thus, the temperature ranges from -190 °C to 110 °C.
Illustration:
Why is the troposphere wider at the equator than at the poles?
Solution:
The thickness of the troposphere varies around the planet. Since the spinning of the Earth tends to shift air towards the equator, the troposphere is thicker near the equator than at the poles. The thickness of the troposphere also varies with season. The troposphere is thicker in the summer and thinner in the winter all around the planet.
Importance of Air:
1. Existing of Living Organs:
Air is most important for human beings, animal, plants etc. All living organs need oxygen in their existence in the environment. They take oxygen from the air in the process of breathing. This oxygen helps to produce energy from the food. In this process carbon dioxide is formed which is breathed out.
2. Maintain a Balance:
Animal and human beings need oxygen from the air and give out carbon dioxide gas to the air. This carbon dioxide used by the plants at the time of photosynthesis and gives out oxygen. In this process nature maintain balance between carbon dioxide and oxygen.
3. Role of Nitrogen:
Nitrogen gas also essential for the growth of plants. Plant cannot absorb directly from the air. This nitrogen gas is converted into nitrates and ammonium salts with the help of bacteria present in the soil. Plant absorbs these to form proteins which are essential for their growth.
4. Act as Shield:
Atmosphere also acts as a shield like an umbrella to protect from the harmful rays of sunlight. Ozone gas which is present in the upper part of the atmosphere, prevent harmful ultraviolet rays of sunlight to reaching the surface of the earth. Thus, ozone layer protects the harmful effects of the ultraviolet rays present in the sunlight on animals and plants.
4. Term Paper on Water: A Precious Resource:
The universal solvent, the existence of life on earth is mainly because of the abundant liquid water. Other planets may have water, but they either have it as a gas (Venus) or ice (Mars). All life on earth is thought to have arisen from water. The bodies of all living organisms are composed largely of water. About 70 to 90% of all organic matter is water.
The chemical reactions in all plants and animals that support life take place in a water medium. Water not only provides the medium to make these life sustaining reactions possible, but water itself is often an important reactant or product of these reactions. Water is a polar covalently bonded molecule. This unequal sharing of the electrons results in a slightly positive and a slightly negative side on the molecule.
Water is a universal, solvent due to the marked polarity of the water molecule and its tendency to form hydrogen bonds with other molecules. In terrestrial living systems, the water is considered as the solvent of overwhelming biological importance. It provides a fluid in which molecules of nutrients and waste products can be dissolved and transported, helps to regulate the temperature and preserves chemical equilibrium within living cells, and makes up a major fraction of the body weight of every organism.
Water is the natural inexhaustible resource which makes life possible. Water is wonderful liquid as it exists in 3 different physical states: namely liquid (water), gaseous (water vapors) and solid (ice). Around 71% of earth is covered with water of which 96.5% is found in sea and ocean (saline) and 2.5% fresh water.
Out of the fresh water found, 1.7% is groundwater, 1.7% is as glaciers and the ice caps of Antarctica and Greenland, and 0.001% is in the air as vapor, clouds (formed of solid and liquid water particles suspended in air), and precipitation. Availability of water varies from place to place.
Availability of fresh water is an important factor for genetic variation in a region. A region with less water or scarce water present is less abundant with different species of plants and animals (ex. desert) than in the region with ample water supply.
Importance of Water:
All cellular processes take place in a water medium. All the reactions that take place within our body and within the cells occur between substances that are dissolved in water. Substances are also transported from one part of the body to the other in a dissolved form.
Hence, organisms need to maintain the level of water within their bodies in order to stay alive. The terrestrial life-forms require fresh water to stay alive because their bodies cannot tolerate or get rid of the high amounts of dissolved salts in saline water. Thus, water sources need to be easily accessible for animals and plants to survive on land.
Factors Affecting pH of Water:
1. The Concentration of Carbon Dioxide in the Water:
Carbon dioxide (CO2) enters a water body from a variety of sources, including the atmosphere, run-off from land, release from bacteria iii the water, and respiration by aquatic organisms. This dissolved CO2 forms a weak acid. Natural, unpolluted rainwater can be as acidic as pH 5.6, because it absorbs CO2 as it falls through the air. Because plants take in CO2 during the day and release it during the night, pH levels in water can change from daytime to night.
2. Geology and Soils of the Watershed:
Acidic and alkaline compounds can be released into water from different types of rock and soil. When calcite (CaCO3) is present, carbonates (HCO3, CO3-2) can be released, increasing the alkalinity of the water, which raises the pH. When sulfide minerals, such as pyrite, or “fool’s gold,” (FeS2) are present, water and oxygen interact with the minerals to form sulfuric acid (H2SO4). This can significantly drop the pH of the water. Drainage water from forests and marshes is often slightly acidic, due to the presence of organic acids produced by decaying vegetation.
3. Drainage from Mine Sites:
Mining for gold, silver, and other metals often involves the removal of sulfide minerals buried in the ground. When water flows over or through sulfuric waste rock or tailings exposed at a mine site, this water can become acidic from the formation of sulfuric acid. In the absence of buffering material, such as calcareous rocks, streams that receive drainage from mine sites can have low pH levels.
4. Air Pollution:
Air pollution from car exhaust and power plant emissions increases the concentrations of nitrogen oxides (NO2, NO3) and sulfur dioxide (SO2) in the air. These pollutants can travel far from their place of origin, and react in the atmosphere to form nitric acid (HNO3) and sulfuric acid (FI2SO4). These acids can affect the pH of streams by combining with moisture in the air and falling to the earth as acid rain or snow.
Very high (greater than 9.5) or very low (less than 4.5) pH values are unsuitable for most aquatic organisms. Young fish and immature stages of aquatic insects are extremely sensitive to pH levels below 5 and may die at these low pH values. High pH levels (9-14) can harm fish by denaturing cellular membranes.
Changes in pH can also affect aquatic life indirectly by altering other aspects of water chemistry. Low pH levels accelerate the release of metals from rocks or sediments in the stream. These metals can affect a fish’s metabolism and the fish’s ability to take water in through the gills, and can kill fish fry.
5. Term Paper on Soil:
Meaning of Soil:
Soil is the mixture of minerals, organic matter, gases, liquids, and the myriad of organisms that together support plant life. It is a natural body that exists as part of the pedosphere. The soil plays a major role in four important factors.
They are as follows:
i. It is a medium for plant growth.
ii. It is a means of water storage, supply and purification.
iii. It is a modifier of the atmosphere.
iv. It is a habitat for organisms that take part in decomposition of organic matter and the creation of a habitat for new organisms.
Soil Formation:
Soil formation starts by disintegrating the rock under the influence of climate. Rain water will dissolve the rock elements, the temperature fluctuations will cause differential expansion and contraction of the rock-forming crystals, and the freezing and thawing of water captured in the rock will widen existing cracks and cavities.
Pioneer vegetation, at first lichens, will settle, and their roots will further loosen the rock. Moreover, decaying plant debris will produce organic acids, which further attack the rock. Organic matter will start to accumulate and be mixed with the mineral material provided by the rock. This is how the soil is formed.
Soil Erosion:
When the soil travels along with air, wind or rain and leaves land with rocks or pebbles is called soil erosion. It is a loss of upper layer of soil.
There are several causes of soil erosion. Natural causes that involve high wind with speed and rains which take away soil with themselves. Many human factors also give rise to loss of soil. Deforestation (cutting of forest), overgrazing, improper agricultural practices, and sloppy areas are reasons that enhance the soil loss.
This results in reduced soil fertility, land sliding, desert formation on land, floods, etc. We can reduce it by growing more trees to hold soil, ploughing at right angles, controlling grazing, growing trees on slopes to avoid loss of soil.
Aspects of Soil:
For practical purposes, the following aspects of soil studies must be considered: 1. Soil Composition 2. Soil Structure 3. Aeration and Water Supply 4. Soil Temperature.
i. Soil Composition:
Weathering processes carried out by rain, sun, wind or frost/ice break down rocks to form soil. Since rocks are so varied in their chemical composition, including such minerals as calcium, potash, phosphorus, iron, aluminium, silica and soda, the soils which result from the weathering of these rocks will also differ greatly. For instance, the weathering of rocks rich in iron may produce soils rich in iron oxides.
On the other hand the weathering of limestone usually produces a soil with little lime content because the lime (calcium) is dissolved and carried away by rain-water. Thus the composition of the soil is greatly dependent on the nature of the parent material, i.e. the rock on which it was developed.
Other factors are also important. Soil is either sedentary, that is the soil may be found near the parent rocks from which it is derived; or it is transported. Soil may be carried to distant lands by winds e.g. loess; by ice, e g. boulder clay; by running water, e.g. silt; or by volcanic eruptions, e.g. volcanic ash.
Apart from their mineral constituents soils also contain organic matter derived from the decomposition of plants and animals. This is known as humus and is renewed by leaf-fall and root decay. The fertility of the soil is often determined by the amount of humus present, for humus improves soil structure, yields plant food and assists in retaining soil moisture.
Crops require varying amounts of the different mineral nutrients in the soil. For example cotton requires soils rich in nitrates, rubber does best on deep, slightly acidic soils and cocoa grows well on soils rich in iron and potassium.
It is necessary to replace the worn-out soil nutrients from time to time if a high level of productivity is to be maintained. Manuring, crop rotation and fallowing are some of the methods used by farmers to keep their soils fertile.
ii. Soil Structure:
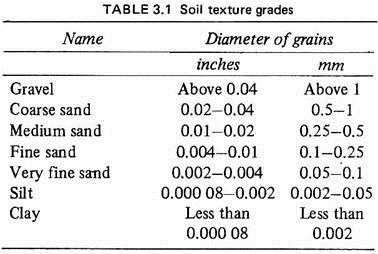
The physical texture of the soil, depending on the size of the soil particles, may be coarse, medium or fine. All soils possess some particles of all these groups but the proportions vary. Thus sandy soils have more coarse particles than fine ones; loams have equal proportions of coarse, medium and fine grains and clays have a large proportion of fine particles (Fig. 3.1; Table 3.1).
The texture of the soil governs such vital aspects of agriculture as ease of ploughing, root penetration and aeration. Clayey soils are very moisture-retentive and are thus heavy to work. They are best suited to wet crops like lowland padi.
On the other hand sandy soils, comprising mainly the coarser grains, lack coherence and are much easier to work. They are better suited to crops like groundnuts, which need greater aeration and cannot tolerate stagnant water.
Soil structure is also affected by the presence in the soil of various materials which bind the individual soil particles together. These binding materials act as a kind of weak cement and their presence makes soil different from loose sand, for example, which would simply run through the fingers. Soil is loosely cemented into small or large crumbs, or lumps.
The greater the tendency to adhesiveness in the soil, the larger these lumps will be so that clay, for instance, is very sticky and lumpy while sandy soils are drier and form only small crumbs. Several materials such as carbonate of lime and oxidized iron (which gives many soils a reddish colour) act as cementing agents. But more important are colloids.
These are very fine particles of clay and humus, less than 1/60,000 cm (1/150,000 inch) in diameter, which bind together the larger grains in the soil. They are most numerous in clays and loams. The presence of colloids in soils also increases their retention of water. The texture of the soil determines the size of pore spaces between particles and lumps in which air and water can circulate.
Factors such as the work of worms, micro-organisms and soil bacteria, or the action of frost can affect soil structure. The constant cultivation of the soil including ploughing, removing large stones and applying mineral fertilizers or organic manure can both change and improve the qualities of soils.
iii. Aeration and Water Supply:
All plants need both air and water to survive. Water is absorbed through the roots and must therefore be present in the soil. It may be supplied by rain-water which sinks into the earth or by irrigation water applied with a hose or sprinkler or led to the fields by means of ditches and canals.
In soils, water is present as a thin film around the particles. This is known as hygroscopic water. It is more abundant in humid than arid regions, more in fine than coarse soils. Water absorption by plants through their root hairs is easiest in well flocculated soil where the pore spaces for air and water penetration form between 35 per cent and 50 per cent of the volume of the soil.
If there is insufficient water in the soil this will prevent the plants taking in mineral nutrients which are dissolved in the groundwater. On the other hand, completely saturated soil or waterlogged conditions may rot the roots of plants. It will also impede the free circulation of air in the soil which is vital for crop survival.
In waterlogged conditions all the pore space is occupied by water so that air cannot move freely in the soil and cannot be absorbed by plants. Waterlogging may result from the formation of a hard pan. This is caused by the accumulation of certain cementing materials such as iron in the sub-soil. Alternatively, in low-lying areas natural drainage may be impeded.
Waterlogging can be relieved by the digging of drainage ditches to allow excess water to escape. Heavy soils can be improved by the addition of lime. Light soils which dry out quickly on the other hand are improved by manuring and by ensuring an adequate supply of water.
iv. Soil Temperature:
The soil is heated by the sun during the day and the effectiveness of this heating varies considerably between tropical and temperate lands and between the ‘sunny’ and the ‘sheltered’ slopes of mountains. All plants need a sufficient amount of heat to be able to germinate and grow. The minimum temperature for plants to grow is 6°C (42° F).
The optimum temperature is the temperature at which plants grow best; temperatures higher or lower either retard growth or make cultivation impossible. The effect of soil temperature on plants is most profound in the temperate lands where the farming year is governed by seasons.
Winter wheat, sown in late autumn, lies through the long, cold winter and germinates when the ground thaws in spring. The crop is ready for harvest in the bright, sunny summer. But further polewards or towards the continental interiors the winter cold is so severe that no wheat can be sown in winter. Sowing can only begin in spring, when the weather conditions are milder, and the crop is harvested in late summer.
A quarter of the world’s wheat comes from such areas and is known as spring wheat. In the northern hemisphere, more fruits and vegetables are grown on the south-facing slopes which receive more of the sun’s heat. The north-facing slopes in the European Alps may be colder by as much as 3° to 6°C (5°-10°F).
Apart from the heat received from the sun, soil temperature is influenced by several other factors. The decay of vegetative matter, when acted on by the microscopic bacteria within the soil, liberates heat. This is one reason why compost or manure is added to the soil. The rate of absorption and radiation of solar energy in different types of soil over different parts of the globe also differs.
Generally speaking, dark-coloured soils absorb more of the sun’s heat than light-coloured ones. In temperate lands this may make a difference of between 3.5° and 5°C (6° and 9°F.) in the soil temperature. Soils also lose heat at night through radiation when the air temperature drops. Bare soils lose heat much faster than those covered with vegetation.
This explains why night frosts do not penetrate so deeply in the grass-covered Steppes as they do in the sandy deserts of the Sahara. The presence of air and water in soil, both of which are poor conductors of heat, also affects soil temperatures.
Thus differences in temperature exist between wet and dry soils, and between compact clay and porous sand. For many garden crops and the more delicate fruits, farmers have to take soil temperature into consideration if the maximum benefit is to be attained.
Factors for Soil Formation:
The factors or processes that make soil:
a. Sun:
The sun heats up rocks during the day so that they expand. At night, these rocks cool down and contract. Since all parts of the rock do not expand and contract at the same rate, this results in the formation of cracks and ultimately the huge rocks break up into smaller pieces.
b. Water:
Water helps in the formation of soil in two ways:
Method 1:
One, water could get into the cracks in the rocks formed due to uneven heating by the sun. If this water later freezes, it would cause the cracks to widen.
Method 2:
Flowing water makes cracks and takes away even a hard rock over long periods of time.
Fast flowing water often carries big and small particles of rock downstream. These rocks rub against other rocks, and the resultant abrasion causes the rocks to break down into smaller and smaller particles. The water then takes these particles along with it and deposits it further down its path. Soil is thus found in places far away from its parent rock.
c. Wind:
In a process similar to the way in which water rubs against rocks and wears them down, strong winds also erode rocks down. The wind also carries sand from one place to the other like water does.
The presence of decayed living organisms in soil is called humus. The production of humus from decaying vegetation debris will equal its consumption by soil microbe, fauna and flora. The transformation of rock minerals into soil minerals will keep pace with the removal of earlier formed soil minerals. Slow surface wash of topsoil is matched by new formation of soil material from the bedrock. The soil has aged.
d. Topsoil:
The topmost layer of the soil that contains humus and living organisms in addition to the soil particles is called the topsoil. The quality of the topsoil is an important factor that decides biodiversity in that area.