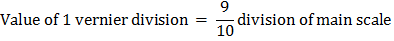ADVERTISEMENTS:
In this article we will discuss about:- 1. Introduction to Hydrosphere 2. Unique Properties of Water 3. Biological Method of Zonation 4. Lotic Environment 5. Lentic Environment 6. Nitrogen and Phosphorus Cycles 7. Complexion in Natural Water and Waste Water 8. Micro-Organic – The Catalyst of Aquatic Chemical Reactions 9. Nitrogen Fixation 10. Eutrophication 11. Water Circulation.
Introduction to Hydrosphere:
ADVERTISEMENTS:
Water is a very complicated and vitally important substance. It is the medium which gave birth to the first primitive living molecules, and without it no life can exist.
Throughout history, the quality and quantity of water available to man have been vital factors in ascertaining his well- being. Whole civilizations have disappeared due to water shortages resulting from changes in climate, and the process continues today with the starvation of African peoples as the Sahara marches southward. Disastrous floods have always plagued mankind.
Waterborne diseases are able to kill millions of people although in the more well-developed areas of the world, the great plagues of cholera and typhoid have been controlled. Ambitious programs of dam and like construction have reduced flood damage, though not without undesirable side effect. However, problems with water remain, and in some respects have been becoming more serious.
Any consideration of environmental chemistry as applied to water needs some understanding of the sources, transport, characteristics, and composition of water. The chemical reactions taking place in water, and the chemical species found in it, get influenced strongly by the environment in which the water is found. The chemistry of water exposed to the atmosphere has been quite different from that of water at the bottom of a lake. Microorganisms play a vital role in determining the chemical composition of water.
Unique Properties of Water:
The study of water is termed as hydrology.
This is divided into a number of subcategories:
ADVERTISEMENTS:
(i) Limnology:
It is the branch of the science which deals with the characteristics of fresh water, including biological properties as well as chemical and physical properties.
ADVERTISEMENTS:
(ii) Oceanography:
It is the science of ocean and its properties.
Water is having a number of unique properties, without which life could not exist. Many of these properties have been due to hydrogen bonding in water. It has been an excellent solvent for many Materials. Thus water is the basic transport medium for the nutrients and waste products involved in life processes.
The extremely high dielectric constant of water compared to other liquids is having a profound effect upon the solvent properties of water, in that most ionic materials are dissociated in water. With the exception of liquid ammonia, water is having the highest heat capacity of any liquid or solid, 1 cal x g–1 x deg–1. Due to this high heat capacity, a relatively large amount of heat is needed to change the temperature of a mass of water appreciably, which can have a stabilizing effect upon the temperature of whole geographic regions.
In addition, it does not allow sudden large changes of temperature in large bodies of water, and thereby protects aquatic organisms from the shock of abrupt temperature variations. The extremely high heat of vaporization of water, 585 cal/g at 20°C, likewise helps to stabilize the temperature of bodies of water and the surrounding geographic regions. It also influences the transfer of heat and water vapour between bodies of water and the atmosphere.
Water is having its maximum density at a temperature (4°C) above its freezing point. The fortunate consequence of that fact has been that ice floats so that few large bodies of water ever freeze solid. Furthermore, the pattern of vertical circulation of water in lakes, a determining factor in their chemistry and biology, governed largely by the unique temperature-density relationship of water.
Biological Method of Zonation:
As sunlight has been such an important factor in the productivity of these systems, it has been customary to divide both marine and freshwater systems into definite zones on the basis of the depth of sunlight penetration. This has been primarily a biological method of zonation. The various wavelengths of light are rapidly filtered out as they pass through a water column.
Based upon the degree of light penetration, it has been possible to divide a system into certain zones. The upper zone of sunlight penetration is known as the euphotic zone. Beneath this is a zone of perpetual darkness, the dysphotic zone, which is extending from the bottom of the euphotic zone to the bottom. The bottom sediment underlying the dysphotic zone is known as the benthic zone and the regeneration zone.
According to this type of classification, shallow lakes, streams, and rivers would not behave as dysphotic zone, as they would be shallow enough to allow for sunlight penetration down to the benthic zone. In addition, the position of both the euphotic and dysphotic zone in deep systems generally shifts with environmental conditions.
ADVERTISEMENTS:
For example, during periods of heavy rainfall, when sediment has been washed into the system, the physical presence of this suspended sediment would tend to block the sunlight, bringing about a reduction of the euphotic zone and an increase of the dysphotic zone.
In typical aquatic and marine systems the euphotic zone has been regarded to be both the zone of sunlight penetration and the zone of oxygen and food production. It has been in this zone that the plants and the primary consumers would be found, at least during the periods of active photosynthesis and grazing. The other consumers (secondary, tertiary, etc.) would occur in all three zones, depending upon the availability of their food source.
Lotic Environment:
The lotic environment is consisting of all inland waters in which the entire water body continually moves in a definite direction. Thus rivers, streams and brooks are regarded to be lotic environments. These systems have been generally originating from precipitation that falls on the earth’s sloping surface and flows downhill as sheet wash. Sheet wash tends to accumulate in low areas and to form intermittent rivulets, which eventually flow into streams.
When the water flow continues, it will tend to erode the bed of the stream, making it to intercept the groundwater table and become a permanent stream. As additional streams are formed and eventually meet, the resulting length, depth, and size of the system will warrant classification as a river.
The rivulet-stream sequence can still get observed upstream, but in an ever-changing position, because erosion will make an upstream migration of these features. The migration will continue until halted by a natural feature like drainage divine. A direct result of this erosion-induced geological migration has been a continual migration of biological habitats. In other words, erosion will make the headwaters to “migrate” upstream which is followed by a similar “migration” of the environmental conditions characteristic of rivulets, streams, and rivers.
The animal and plant communities that occupy each of these habitats must also move upstream, to remain in the same habitats (which has been in a constantly changing position because of erosion), or must adapt to the gradually changing conditions brought about by the erosion caused by the running waters. In natural, undisturbed systems these changes have been very slow and permit ample time for the organism to adapt to the changing conditions.
As lotic systems tend to be shallower and narrower than lakes, there has been generally a greater proportion of water exposed to land surfaces. Consequently, streams have been more intimately associated with and affected by the terrestrial environment than have been most lentic systems. In addition, lotic systems have been more dependent on the surrounding land areas for a large proportion of their nutrient supply.
Lentic Environment:
The lentic environment has been including all inland waters in which the water has been not continually flowing in a definite direction. The water in these systems have been essentially standing, although some water motion may take place because of wind driven waves and or in the vicinity of inlets and outlets. From a long-term geologic viewpoint, however, these inlets and outlets have been regarded to be temporary impoundments that will in time disappear, converting the system to a flowing-water environment. Typical lentic environments have been regarded to be lakes, ponds, and marshes.
In lentic systems, the aging process has been just the reverse of that described for lotic systems. Whereas streams tend to get wider and deeper as they age, lakes tend to get shallower and the banks extend into what was originally open water. Natural filling has been generally due to wind-blown materials (sand, leaves, etc.) entering the system, sediment input by streams, and terrestrial runoff and aquatic plant and animal debris. Not all lentic environments have been becoming shallower by this type of filling alone. However, in many cases outlets may widen and deepen, making an increased out flow of water having a subsequent lowering of the water level.
Lakes could be generally classified, on the basis their depth and nutrient levels, as oligotrophic, eutrophic, or senescent.
An oligotrophic lake has been regarded to be in its young stage. These lakes have been very deep, with a sand or rock bottom, and have been low in nutrients. Because of the paucity of nutrients, both plants and animal life are low.
Eutrophic lakes have been regarded as middle-aged systems. They have been relatively shallow in comparison to oligotrophic systems, with a silty or mud bottom, and have sufficient nutrients for supporting a large population of plants and animals.
A senescent lake has been in the oldest stage of development. The bottom sediments in senescent system have been consisting of a thick layer of organic silts and/or muds, nutrient levels are high, and the system has been very shallow. There has been a large percentage of rooted emergent vegetation which is growing throughout the system. Terrestrial or marsh vegetation tends to grow along the banks and into the lake itself over the root mat.
On the basis of temperature (and thus, indirectly, density) lake could be divided into three distinct zones – the epilimnion, the thermocline, and the hypolimnion. The epilimnion has been the upper zone of gradual temperature change. Below the epilimnion has been a zone of rapid temperature change, termed the thermocline. In order for this region to meet the established criterion for classification as a thermocline, the temperature must alter by at least 1°C for every meter of depth.
If the temperature changes at a lesser rate relative to depth, these water would be classified as a portion of the epilimnion. Below the thermocline there has been an area of water that has been constantly at a temperature of 4°C, termed the hypolimnion. As the waters of the hypolimnion have been at 4°C they have been at their maximum density and will occur at the bottom layer in any system.
Comparing the relative water temperatures and density variations seasonally, it has been noted that in summer the warmer water will get located in the upper epilimnion and the colder, denser, water will be located in the hypolimnion. In the winter the colder; less dense water at temperatures below 4°C will be located in the hypolimnion. In either case, the less dense water has been in the epilimnion and the dense (4°C) water has been always found in the bottom waters of the hypolimnion.
Nitrogen and Phosphorus Cycles:
All systems need two major chemical components for the growth, reproduction, and general metabolic processes of the organisms that inhabit those systems. These components, termed essential elements, have been phosphorus and nitrogen. Plants need phosphorus and nitrogen in their dissolved form as inorganic phosphate (termed orthophosphate and abbreviated o-PO4) and inorganic nitrate (NO3).
Although animals also need phosphorus and nitrogen, they need it in its organic form as organic phosphorus and organic nitrogen (generally obtained in the form of amino acids or protein by grazing on plants or predation on other consumers). Should animals attempt to ingest phosphate or nitrate in its inorganic form, it would be totally unusable and immediately excreted.
Phosphorus and nitrogen tend to cycle in systems, passing from plant to various animal communities, then into their inorganic forms, and then back into the plant components of the system. The discussion of these cycles is conveniently begun with those plants which will take in the inorganic materials.
During the process of photosynthesis the plants are able to convert o-PO4 and NO3 into their organic forms like nucleic acids (DNA, RNA), amino acids, and proteins, which contain various ratios of phosphorus and nitrogen in their molecular structures. These materials are used by the plants in their metabolic process and stored as new plant tissue during the growing season.
Herbivores (primary consumers) get their phosphorus and nitrogen by feeding directly on the plants in the system, while the higher consumers obtain their phosphorus and nitrogen by predation. At each step in the food chain some of the material gets lost to the next link by death and/or excretion. In either case the phosphorus and nitrogen present in the excretory matter or the bodies of the plants and/or animals not consumed will sink through the water column and enter the benthic zone.
Although bacterial decomposition presumably starts from the moment of excretion or at death, the majority of the decomposition occurs in the benthic sediments (regeneration zone). It has been in the regeneration zone that bacterial action converts the organic nitrogen and phosphorus into their inorganic form. It has to be noted that phosphorus readily gets converted into its inorganic form.
Plants inhabiting any system need nitrogen and phosphorus in certain ratios. The ratios needed by plants in both terrestrial and freshwater systems have been extremely variable and thus are dependent upon the specific populations of plants that inhabit a particular system. Since, however, plant communities do require not only phosphorus and nitrogen but need these materials in certain ratios, it is the availability of these nutrients in their proper ratio that becomes important from the viewpoint of productivity.
As these ratios have been so variable in freshwater systems, it has been preferable to cite a hypothetical example. Assume that in a given freshwater system it has been determined that the proper phosphate/nitrate ratio is 1.8. This would reveal that (at least in this system) the plants need one phosphate for every eight nitrates, and that during the periods of active plant growth and metabolism, the plants would be removing phosphate and nitrate from the water in a 1:8 ratio and tying it up, as plant tissue, in this ratio.
The consumers, too, would be getting the same phosphorus/nitrogen ratio. When these organisms died and fell to the regeneration zone, the decomposers would, ultimately, return this material to the water column in this ratio.
In a system in which the producers need a 1:8 ratio of phosphate to nitrate, should the ratios become disturbed (1 : 16, for example) the plants would tend to remove 8 nitrates and all phosphate. The remaining 8 nitrates could not be used as there would not be sufficient phosphate in the system. In this case the factor limiting further plant growth would be phosphate. If the situation has been reversed and there were a 2 : 8 ratio, the limiting factor would be the lack of additional nitrates. In this case the plants would be able to remove all the nitrate but, since the nitrates were present in the lesser ratio, they would only be able to use one of the phosphates.
In many studies it is advisable to not only measure the inorganic phosphate and nitrate present in a given system, but also to determine the amount of nitrate, ammonia, and organic phosphate. By performing these additional analyse, not only can the productivity at the time of sampling by determined, but also the potential productivity in a given system can be inferred.
In the majority of freshwater systems it is found that phosphate has been generally the limiting factor. This has been due to the presence of blue green algae which have been common in most freshwater systems. These forms have the ability to fix atmospheric nitrogen, thus increasing, the concentration of nitrate in the water column. Apparently the normal phosphate input into these systems from the weathering of phosphatic terrestrial materials is insufficient to counterbalance the amount of nitrogen input by the blue-green algae.
Both lentic and lotic systems, are having the same basic mechanisms of nutrient cycling and sunlight relationships as explained above. Beyond these commonly shaded characteristics, however, the systems very greatly and should be considered as separate and discrete entities.
Complexion in Natural Water and Waste Water:
Naturally occurring chelating agents like humic acids and amino acids are found in natural water and soil. The abundance of chloride in sea water gives rise to the formation of some chloro-complexes. There are synthetic chelating agents, like sodium tripolyphate, sodium ethylenediamine tetracetate (EDTA), sodium nitiriotriacetate (NTA) and sodium citrate, which are discharged in small amounts into aquatic system from different industrial wastes.
The ligands occurring in natural water and waste water are having a variety of organic groups which are able to co-ordinate to metal ions:
These ligands are known to form complexes with most metal ions (Mg2+, Ca2+, Mn2+, Fe3+, Cu2+,Zn2+,CO2+, Ni2+, Sr2+, Cd2+ and Ba2+) which are present in natural water and biological system.
Humic Substances:
These are the complexing agents which are important and found in nature. Although their existence has been known since 1800, yet their structural and chemical properties have been still challenging chemists.
Humic substances have been non-degradable materials which are formed during the decomposition of vegetation. They are found as deposits in soil, marsh sediments and any location where there have occurred large quantities of vegetation.
They are generally classified on the basis of solubility. If a humic substance could be extracted with a strong base and the resulting solution acidified the products have been- (a) a non- extractable plant residue termed as humin, (b) a material which gets precipitated from the acidified extract, termed as humic acid and (c) an organic material which is left in the acidified solution, called fulvic acid. The properties of water have been mainly affected by the humic substances, both soluble and insoluble because of their acid-base adsorptive and complexing properties. The soluble fulvic acid is having an effect on the properties of water, while the insoluble humin and humic acids are able to alter water quality by involving exchange of cations, organic materials, etc., with water.
The elementary composition of humic substances, C, 45 – 55%; O, 30 – 45%; H, 3-6%; N, 1-5% and S, 0-1% ‘Humin’, humic acid, and ‘fulvic acid’, are not applicable to single compounds but to a wide range of compounds. In general, humic substances constitute the high molecular-weight polyelectrolytic macromolecules whose molecular weights range from a few hundred (fulvic acids) to tens of thousands (humic acid and humin fractions).
They are having a carbon skeleton with a high degree of aromatic character and a large percentage of functional groups having oxygen. They may also be having protein-like materials and a carbohydrate fraction. These fractions can readily get hydrolyzed from the aromatic nucleus which is able to withstand chemical and biochemical attack.
When these occurs the decomposition of humic acid, the following typical decomposition products are obtained:
The hypothetical structural formula of fulvic acid may be put as follows:
The fulvic acid is having a formula weight 666 and chemical formula, C20H15 (COOH)6(OH)5 (CO)2.
Humic substances are able to chelate metal ions through a carboxyl and a phenolic hydroxyl group.
Insoluble humic substances such as the humic and humic acids are able to exchange cations with water and are having the capacity to accumulate large amounts of metals.
Micro-Organic – The Catalyst of Aquatic Chemical Reactions:
Micro-organisms such as the bacteria, fungi, and algae have been living catalysts which are able to being about a vast number of chemical processes in water and soil. A majority of the important chemical reactions taking place in water, particularly those involving organic matter and oxidation-reduction processes, take place through bacterial intermediaries. Algae constitute the primary produces of biological organic matter (biomass) in water.
Micro-organisms have been considered to be responsible for the formation of many sediment and mineral deposits. Algae photosynthesis increases the pH of water thus causing the formation of calcium carbonate deposits. Some anaerobic bacteria produce hydrogen sulfide which reacts with metal ions to produce sulfide mineral deposits. With present technology, micro-organisms are able to play the dominant role in secondary waste treatment.
Pathogenic micro-organism must be eliminated from water purified for domestic use because the major epidemics of typhoid, cholera, and other water borne diseases have been resulted from pathogenic micro-organisms in water supplies. There is some speculation that viruses in water escape conventional water treatment and cause respiratory and digestive tract diseases, hepatitis, and perhaps even some forms of cancer.
Types of Micro-Organisms in Waters:
In considering aquatic chemistry, micro-organisms may be included in the three categories of bacteria, fungi, and algae. Fungi and bacteria (with the exception of photosynthetic bacteria) are termed as reducers. Reducers break down chemical compounds to more simple species, and thereby extract the energy needed for their growth and for their metabolic needs. As reducers can utilize only chemical energy, any chemical transformation mediated by them must involve a net loss of free energy. However, compared to higher organisms, the energy utilization of bacteria and fungi has been very efficient.
Algae have been classified as producers, as they use light energy and store it as chemical energy. In the absence of sunlight, however, algae use chemical energy for their metabolic needs. In a sense, therefore, bacteria and fungi may be looked upon as environmental catalysts, while algae are aquatic “solar fuel cells.”
Algae may be regarded as generally microscopic organisms which subsist on inorganic nutrients and produce organic matter from carbon dioxide by photosynthesis. The general nutrient requirements of algae are carbon (from CO2 or HCO3–), nitrogen (generally as NO3–), phosphorus (as some form of orthophosphate), sulfur as SO42–), and trace elements such as sodium, potassium, calcium, magnesium, iron, cobalt, and molybdenum.
In a highly simplified form, the production of organic matter by algae photosynthesis may be put by the equation–
Where, CH2O denotes a unit of carbohydrate and hν stands for the energy of a quantum of light. Fogg (1) has represented the overall formula of the algae, Chlorella, as C5.7H9.8O2.3N (including phosphorus the formula would be C5.7H9.802.3NP0.06).
Using Fogg’s formula for algae biomass, the overall reaction for photo-synthesis may be the following:
In the absence of light, algae are able to metabolize organic matter in the same manner as do non-photosynthetic organisms. Thus, algae may be able to satisfy their metabolic demands by using chemical energy from the degradation of stored starches or oils, or from the consumption of algae protoplasm itself In the absence of photosynthesis the metabolic process uses oxygen, so that during the hours of darkness an aquatic system having a heavy growth of algae may get depleted in oxygen.
Microbially Mediated Redox Reactions:
Bacteria are able to mediate a variety of redox reactions and thereby drives the energy is able to for their metabolic processes and reproduction.
Some environmentally important redox reactions of this category are given as follows:
Bacteria have been taking fact in many biogeochemical processes in water and soil and in many important elemental cycles in nature, such as those of nitrogen, carbon and sulphur. They help in the formation of many mineral deposits including some of those of iron and manganese. Bacteria have been also instrumental in the formation of iron and manganese deposits in natural water systems and pipes used for transporting water.
Nitrogen Fixation:
The biochemical pathway of nitrogen fixation by which atmospheric N2 is chemically bound in biological or simple inorganic compounds is not simple, and a number of conflicting theories have been advanced to explain the mechanisms of the process. Tracer studies of this vital process by which atmospheric nitrogen gets incorporated into living matter revealed that the first detectable fixed nitrogen species has been NH3.
The ammonia has been then converted rapidly to glutamic and aspartic acids by involving the following reactions:
Few kinds of aquatic micro-organisms are having the ability to fix atmospheric nitrogen. Among the aquatic bacteria which are having this capability have been photosynthetic bacteria Azotobacter, and several varieties of Clostridium. Among the algae, blue-green algae are able to fix atmospheric nitrogen. In most natural fresh water systems, however, the fraction of nitrogen fixed by organisms in the water relative to that originating from the decay of organic material, fertilizer runoff, and other external sources, has been quite low.
The best known and most important form of nitrogen-fixing bacteria has been Rhizobium, which has a symbiotic (mutually advantageous) relationship with leguminous plants such as clover or alfalfa. The Rhizobium bacteria occur in root nodules, special structures attached to the roots of legumes. These bacteria are able to fix atmospheric nitrogen in a chemical form which the legumes can utilize.
When the die and decay, some of the nitrogen gets converted by micro-organisms in the decay process to nitrate ion, which may be utilized by other plants. Before the advent of commercial chemical fertilizers, the growth of leguminous crops was widely used as a means of building soil fertility. Some of the nitrate produced by the decay of legumes enters natural waters. In an indirect way, therefore, Rhizobium bacteria have been responsible for the introduction of some nitrogen into waters.
Nitrification, the conversion of N(-III) to N(V), has been a very common and extremely important process in water and in soil. As it is known aquatic nitrogen in thermodynamic equilibrium with air has been in the form of NO3 ion, N(V), whereas in most biological compounds nitrogen occur as N(-III), such as —NH2 in amino acids. The equilibrium constant for the overall nitrification reaction, written as one electron-mole,
is 107.59 so the reaction is highly favored from a thermodynamic viewpoint.
One of the most important ramifications of this conversion has been that nitrogen gets absorbed by plants primarily as nitrate. When fertilizers are applied in the form of ammonium salts or anhydrous ammonia, a microbial transformation to nitrate enables maximum assimilation of nitrogen by the plants.
If extensive aeration has been allowed to take place in the activated sludge sewage treatment process the ammonia nitrogen in the sewage is largely oxidized to nitrate ion. As the sewage sludge settles out in the settler, the bacteria in the sludge use this nitrate as an oxygen source producing N2 gas. The bubbles of nitrogen gas bring about the sludge to rise, so that it does not settle properly. This can hinder the proper treatment of sewage through carryover of sludge into effluent water.
In nature, nitrification gets catalyzed by two groups of bacteria, Nitrosomonas and Nitrobacter. Nitrosomonas bacteria cause the transition of ammonia to nitrite-
whereas Nitrobacter mediate the oxidation of nitrite to nitrate-
Both of these highly specialized types of bacteria are obligate aerobes, that is, they function only in the presence of molecular O2. These bacteria are also chemolithotrophic, implying that they can use oxidizable inorganic materials as electron donors in oxidation reactions to yield needed energy for metabolic processes. A majority of chemolithotrophic bacteria use CO2 as a carbon source in the synthesis of their biomass and have been therefore, autotrophic. There have been several classes of chemolithotrophic bacteria besides the nitrifying bacteria. These other classes include hydrogen bacteria, colorless sulfur bacteria, and iron bacteria.
For the aerobic conversion of ammoniacal nitrogen to nitrite ion at pH 7.00, the free energy change is – 10.8 kcal.
The free energy change for the aerobic oxidation of one electron mole of nitrite ion to nitrate ion has been — 9.0 kcal.
Both steps of the nitrification process involve an appreciable yield of free energy. It has been interesting to note that the free energy yield per electron mole has been approximately the same for the conversion of NH4+ to NO2– as it is for the conversion of NO2– to NO3–, about 10 kcal/electron moles.
The general term “nitrate reduction” means the microbial processes by which nitrogen in chemical compounds gets reduced to lower oxidation states. In the absence of free oxygen, nitrate may be used by some bacteria as an alternate electron receptor. The most complete possible reduction of nitrogen in nitrate ion involves the acceptance of 8 electrons by the nitrogen atom with the consequent conversion of nitrate to ammonia (+V to -III Oxidation state).
Nitrogen is an essential component of protein, and any organism which utilizes nitrogen from nitrate for the synthesis of protein must first reduce the nitrogen to the – III oxidation state (ammoniacal form). However, incorporation of nitrogen into protein generally has been a relatively minor use of the nitrate undergoing microbially mediated reactions, and has been more properly termed nitrate assimilation.
Generally when nitrate ion functions as an electron receptor, the product has been NO2–. When nitrate ion acts as an electron receptor, the free energy yield per electron mole has been only about 2/3 of the yield when oxygen is the electron receptor.
However, nitrate ion is a good electron receptor in the absence of molecular oxygen. One of the factors limiting the use of nitrate ion in this function has been the relatively low concentration of NO3 – in most waters. Furthermore, nitrite, NO2–, has been relatively toxic and tends to inhibit the growth of many bacteria after building up to a certain level.
Sodium nitrate has been sometimes used as a “first aid” treatment in sewage lagoons which have become oxygen-deficient. It provides an emergency source of oxygen to re-establish normal bacterial growth.
Denitrification:
An important special case of nitrate reduction has been denitrification, in which the reduced nitrogen product has been gaseous N2. At pH 7.00 the free energy change per electron mole of reaction –
is —2.84 kcal. The free energy yield per mole of nitrate reduced to N2 (5 electron moles) has been higher than that for the reduction of the same quantity of nitrate to nitrite. More important, however, the reduction of a nitrate ion to N2 gas consumes 5 electrons, compared to only 1 electron for the reduction of NO3– to NO2–.
Denitrification has been an important process in nature. It is the mechanism by which fixed nitrogen gets returned to the atmosphere. Denitrification is also useful in advanced water treatment for the removal of nutrient nitrogen.
The water is treated with a minimum amount of methanol under anaerobic conditions, and N2 gas is evolved according to the following reaction:
As nitrogen gas is a nontoxic volatile substance which does not inhibit microbial growth, and as nitrate ion is a very efficient electron acceptor, denitrification allows the extensive growth of bacteria under anaerobic conditions.
The Nitrogen Cycle:
The relationships on nitrogen transformations of bacteria may be summarized in the nitrogen cycle. The nitrogen cycle describes the dynamic processes through which nitrogen gets interchanged among the atmosphere, organic matter, and inorganic nitrogen compounds. It is one of nature’s vital dynamic processes.
Iron and Manganese Bacteria:
Some bacteria, including Ferrobacillus, Gallionella, and some forms of Sphaerotilus, use iron compounds in getting energy for their metabolic needs. The bacteria are able to catalyze the oxidation of iron (II) to iron (III) by molecular oxygen –
The carbon source for some of these bacteria is CO2. As they do not need organic matter for carbon, and because they derive energy from the oxidation of inorganic matter, these bacteria may be able to thrive in environments where organic matter is absent.
The microorganism mediated oxidation of iron (II) has been not a particularly efficient means of energy for metabolic processes.
For the reaction, the change in free energy is almost 10 kcal/electron-mole. It has been estimated that approximately 220 g of iron (II) would be needed to produce 1.0 g of cell carbon. The calculation assumes CO2 as a carbon source and a biological efficiency of 5%. The production of only 1.0 g of cell carbon would produce approximately 430 g of Fe(OH)3. It is evident that large deposits of hydrated iron (III) oxide form in areas where iron oxidizing bacteria are able to thrive.
Some of the iron bacteria, notably Gallionella, secrete large quantities of hydrated iron (III) oxide in the form of intricate branched structures. The bacterial cell grows at the end of a twisted stalk of the iron oxide. Electron micrographs of individual cells of Gallionella taken with an electron microscope have shown that the stalks have been composed of a number of strands of iron oxide secreted from one side of the cell.
At pH values near neutrality, bacteria deriving energy by mediating the air oxidation of iron (II) must compete with direct chemical oxidation of iron (II) by O2. The latter process has been relatively rapid at pH 7. As a consequence, these bacteria tend to grow in a narrow layer in the region between the oxygen source and the source of iron (ll). Hence, iron bacteria are sometimes termed as “gradient organism,” and they grow at intermediate pE values.
Bacteria have been strongly involved in the oceanic manganese cycle. Manganese nodules, a potentially important source of manganese, copper, nickel, and cobalt give rise to different species of bacteria which mediate both the oxidation and reduction of manganese. These reactions are enzymatic in nature and get influenced by seawater cations, especially Ca2+ and Mg2+.
Eutrophication:
The term “eutrophication” has been derived from Greek word which means “well nourished”. It describes a condition of lakes or reservoirs involving excess algal growth, which may eventually cause their destruction. The first step in eutrophication of a body of water has been an input of nutrients from watershed runoff or sewage. The nutrient rich body of water gives rise to a great deal of plant biomass by photosynthesis, and a smaller amount of secondary animal biomass.
Deal biomass gets accumulated in the bottom of the lake, where it partially decays, recycling nutrient carbon dioxide, phosphorus, nitrogen, and potassium. If the lake has been not too deep, bottom rooted plants begin to grow, accelerating the accumulation of solid material in the basin. Eventually a marsh gets formed, which finally fills in to produce a meadow or forest.
The eutrophication process has been not a new phenomenon. It has been basically responsible for the formal ion of huge deposits of coal and peat. However, man’s activities are able accelerate eutrophication. Table 2.2 shows the chemical elements needed for plant growth. Most of these occur at a level which is more than sufficient to support plant life in the average lake or reservoir.
Hydrogen and oxygen are provided by the water itself. Carbon is provided by CO2 from the atmosphere or from decaying vegetation. Sulfate, magnesium, and calcium normally occur in abundance from mineral strata in contact with the water. The micronutrients are needed at only very low levels (for example, approximately 40 ppb for copper).
Therefore, the nutrients most likely to be limiting have been the “fertilizer” elements – nitrogen, phosphorus, and potassium. These all occur in sewage and are of course found in runoff from heavily fertilized fields. They are also constituents of various kinds of industrial wastes. Each of these elements can come from natural sources phosphorus and potassium from mineral formations, and nitrogen fixed by bacteria, blue-green algae, or discharge of lightning through the atmosphere.
Generally the plant nutrient most likely to be limiting has been phosphorus, and it has been generally named as the culprit in excessive eutrophication. Household detergents constitute a common source of phosphate in wastewater, and eutrophication control has concentrated upon eliminating phosphates from detergents, removing phosphate at the sewage treatment plant, or preventing phosphate-laden sewage effluents (treated or untreated) from entering bodies of water.
In some cases nitrogen or even carbon constitute limiting nutrients. Particularly in sea water nitrogen may be a limiting nutrient.
The whole eutrophication picture has been a complex one and continued research is required to solve the problem. It is indeed ironic that in a food-poor world, nutrient rich wastes from over fertilized fields or sewage are bringing about excessive plant growth in many lakes or reservoirs. This illustrates again that in general there has been no such thing as a pollutant, there have been only resources (in this case plant nutrients) gone to waste.
Water Circulation:
The three great water reservoirs of the earth – the seas, the continents and the atmosphere are not in any sense self-contained units but are in constant communication with one another. The relationships take the form of a circulation. Under the influence of solar radiation, water is constantly vaporized and emitted into the atmosphere in the form of water vapour. This process can occur in a great variety of ways. On the one hand, water evaporates directly from the surface of seas lakes and rivers, from glaciers and snowfields, or straight out of the ground.
On the other hand, in the process of breathing, all living organisms, animals and plants alike, emit both carbon dioxide and water vapour into the atmosphere. A third process is the emission of water vapor by the combustion of organic materials such as wood, coal and oil, which are to be found all over the earth. Easily the highest proportion of vaporized water emanates directly from the oceans, however.
The water suspended in the atmosphere in the form of water vapour is of the greatest importance to the climate conditions on earth. It conditions the composition of the air masses, exerts an influence on the energy conditions of the atmosphere, and substantially governs and sustains the water circulation.
The water content in the atmosphere is measured in terms of the relative humidity of the air. Whereas some portions of the vaporized water masses remain permanently suspended in the atmosphere in the form of moisture, others are condensed back by the cooling down of ascending airstreams and emerge in the form of clouds, fog, rain or dew. If the water cools still further, snow or hailstones may build up in the clouds. When the clouds get too heavy, precipitation begins and the moisture returns to the earth in the form of rain, snow or hail.
If the precipitation goes straight into the sea or into lakes having no outlet, the water circulation will be complete. If it falls on land, some of it will accumulate by surface drainage in brooks, streams and rivers and so return to the ocean. Other forms of precipitation seep into the earth and replenish the groundwater, or if retained at an upper ground level as trap water will evaporate from there. The trap water is also available to sustain plant life, which absorbs it through its roots and returns it to the atmosphere as water vapour by respiration and transpiration.
This circulation, like all others on the earth, cannot occur in the absence of some motive force. The entire cycle is maintained by the regular inflow of solar energy. Of the total amount of energy absorbed by the earth, about one-third is utilized in maintaining the water circulation. The temperature, humidity and movement (wind) in the atmosphere together determine the level of evaporation.
Although the total volume of atmospheric moisture is very small in comparison with the total hydrosphere, owing to the circulation of the atmosphere an enormous amount of water movement takes place in the course of a year. To produce the annual precipitation on the earth of about 470,000 cubic kilometers, the water in the atmosphere (about 12,300 cubic kilometers) must be replaced 35 to 40 times a year.
Composition of Seawater:
Seawater is a complex solution of various salts, trace elements and gases. There is a measure of equilibrium between the input of soluble matter by the rivers, elutriation (or removal of suspended particles) from the atmosphere by rain, and the loss by precipitation and absorption of nutrients.
The bulk of the soluble matter is washed into the sea through the weathering and erosion of rocks from the solid crust of the earth. Much of this matter is soon precipitated to form new rock deposits on the seabed, which become stratified over geological periods and can form new mountains again as a result of pressure movements in the earth’s crust.
In shorter periods, variations can be caused in the composition of sea-water by biological processes (occasioned by the temperature, for instance) and today in particular by the deposit of waste and toxic substances in the sea. In general, the quantity of inorganic material dissolved in seawater amount to 35 grams per kilogram of water. This represents a solution of 3.5%.
Seawater contains the following elements (average concentration):
Most of these elements are present in seawater in the form of salts, and a smaller portion in the form of dissolved gases. The most important of these are oxygen and carbon dioxide. The oxygen content fluctuates between 0 and 8-5 milliliters per liter. The high concentrations occur near the surface of the water, where the oxygen is in equilibrium with the oxygen in the atmosphere. A second source of the oxygen dissolved in the sea comes from the photosynthesis of phytoplankton, the drifting oceanic plant life.
At great depths it may happen that (owing to the intense activity of bacteria and animals that absorb oxygen) the oxygen content of the seawater falls to zero. In many places, however, owing to the downward trend of the convection currents, a large quantity of dissolved oxygen from the surface water is carried to considerable depths in the sea.
An important part is played by carbon dioxide gas, which is present in considerable quantities in seawater. Since the basic ions of sodium, potassium and calcium are also present in large quantities in seawater, this facilitates the solution of a relatively high percentage of carbon dioxide. Carbon dioxide is one of the basic substances for photosynthesis. The plants living in the sea (especially plankton) take the carbon dioxide they need for photosynthesis directly out of the surrounding water.
The second important effect of carbon dioxide in seawater is its function as a buffer (dissolved matter which keeps the pH value of the solution practically constant over wide areas). Normally the pH value of seawater lies between 7.5 and 8.4. As carbon dioxide and its associated marine chemicals are in equilibrium, the pH value of seawater is approximately constant as regards the input of both acids and bases.
The other gases from the atmosphere are dissolved in seawater in much lower concentrations than oxygen and carbon dioxide.
The concentrations are as follows:
A particular phenomenon is the enrichment of seawater by trace elements that occur in considerable concentrations in living organisms. An example is provided by the ascidian (a tunicate), a small, mostly clinging animal that increases the element vanadium in seawater up to 50,000 times its normal concentration. It is known today that many other trace elements such as iodine, arsenic, nickel, zinc, titanium, chromium and strontium are also very much increased in the water by decaying organisms. Certain fish are known to contribute chromium, nickel, silver, tin and zinc.
Similarly, some of the substances produced by humans get into the sea as waste substances. As such a buildup of substances in the food web takes some time, the damage caused by them is often not noted until too late. The increase of chlorinated hydrocarbons, for instance (especially pesticides such as DDT), reaches its maximum concentration in fish only after a period of 11 years from its production.
Also of great importance in its effects on life in the sea is the content of ammonium compounds, nitrates and phosphates. As these are absorbed by plants to build up their bodies, their content in seawater varies considerably according to the presence of plant life.
Groundwater:
A large part of the precipitated water (rain, snow, dew, etc.) sinks into the ground and supplements the underground water reserves. To be specific, two forms of subterranean water supplies can be distinguished – water that percolates through from the surface, and groundwater proper. Where the rainfall is light, the precipitation penetrates by seepage only into the upper strata and remains suspended in the highest levels. This is particularly evident when the ground consists of peat or other very clayey material.
The highest stratum is then described as a water retentive zone. If the precipitation is greater or the subsoil is very sandy or gritty, the seepage water penetrates the upper strata and joins the groundwater. In the lower lying underground strata the seepage water spreads mostly over an impenetrable rock layer and so provides the groundwater. The water-carrying ground layers are described as groundwater layers.
Particularly rich in groundwater are the underground strata of sand and grit, which mostly emanate from the Pleistocene epoch, as many small fissures open up between the individual soil particles; these fissures can be filled with water.
Connecting lines between groundwater levels of the same size are commonly described as groundwater equivalents. From these it is seen that the groundwater is in constant interchange with the rivers.
As a general rule the groundwater level is higher than the neighboring riverbed. The groundwater layer follows the line of the riverbed, though considerably more slowly, as the river flows downstream, and forms a reservoir for the river; when the river water level is low it is replenished from the groundwater, and when the water level is high it releases surplus water to the groundwater reserves.
In dry regions the groundwater lies considerably deeper than in wet regions. The seepage-water zone contains only ground moisture and as occasion offers also groundwater, which overcomes the force of gravity to rise by capillary attraction. As a result of the evaporation of the capillary waters on the surface, the minerals dissolved in the water are precipitated as salt and gypsum at on in the highest level.
In the bedrock a distinction can be made between quarry water and groundwater. Quarry water seeps through small hollow “reamers”, or holes, in the bedrock, and serves gradually to enlarge them by physical and chemical processes. The chemical processes are particularly marked with a salt and gypsum subsoil or a limestone rock, owing to the high carbon dioxide content of the seepage water.
Ground-stratum water is found primarily in sedimentary rock such as sandstone and loess. If impermeable strata are above or below, big subterranean water systems can be formed that can be tapped by artesian wells.
As a rule, groundwater is very rich in soluble matter. The concentration depends on the duration of the subterranean flow of water, the temperature, the solubility and composition of the rocks. Owing to the usually low oxygen content, oxidation processes occur in these layers only to a limited extent, so that the solubility of many compounds is retained. If such waters come again to the surface to be combined with oxygen, the dissolved compounds will be precipitated as oxides.
Heating of Rivers:
Most power stations use river water for cooling purposes. This can give rise to far-reaching adverse ecological changes. The main problems are a reduction in the self-purification capacity of the river and the danger to river fauna caused by the heating of the river water, the extermination of water organisms in the condenser in the power station, and the climatic effects of heating of the waters.
By the heating of river waters, the dissolving power for the oxygen that is vital to the needs of water organisms is lowered, and at the same time the metabolic processes of the water organisms are accelerated. A higher consumption of oxygen is thus accompanied by a reduction in the supply. This can result in an impairment of self-purification capacity, in the disappearance of oxygen (with the consequent extinction of water organisms and the possible extermination of fish life) and, in extreme cases, in the complete disruption of the water biology.
The heating of river waters leads to a sharp increase in bacteria. One result of this is that in addition to the physical reduction in the oxygen content of the water already caused by the rise in temperature, the remaining oxygen is consumed more quickly. The water acquires a musty smell and taste, which can seriously complicate the purification of drinking water from the river concerned. The bacterial cultures that develop can settle in quiet spots in the river, such as backwaters beside weirs, and putrefy there.
As this problem was recognized fairly early on, authorities imposed limits on the extent to which river water might be heated. These limits have been set too high, however, as they only take account of the physical factors present in clean rivers. The limits laid down stipulate that water temperatures may not be raised by more than 5°C (41 °F), so that the upper limit of 28°C (82°F) is never exceeded.
This threshold value takes no account of the fact that this range of temperature can give rise, in polluted rivers, to extensive disturbances in ecological processes to the point of complete disruption. Detailed investigations have been made into the matter on the river Main in West Germany, from which it emerges that once the level of 23°-24°C (73°-75°F) is overstepped, organisms die from lack of oxygen caused by pollution and heating of the water.
A further problem consists in the sharp increase in temperature of the water drawn into the condenser at a power station, possibly to 40°C (104°F). This is precisely the optimum temperature for pathogenic bacteria such as typhus and enterococci (intestinal bacteria). In clean cold water these disease germs do not multiply, but in polluted warm water well supplied with nutrients they do so freely.
Another serious factor is that when the water heats to 40°C in the condenser, the animals that prey on bacteria rotifers (wheel animalcules) and ciliates (slipper animalcules, etc.) die off. An increase in pathogenic bacteria is thus matched by the extermination of the creatures that prey on them. In a 1000 megawatt power station, about 40,000 liters of water per second are absorbed and heated, which seriously impairs the self-purification capacity of polluted rivers.
A third problem arises from the influence on the climate of heating the river. Scientists at the Nuclear Research Center in Karlsruhe, Germany, have investigated this thoroughly as regards the Rhine. They reached the conclusion that with a rise in temperature of no more than 3°C (37.4°F) the quantity of additional water evaporated from the Rhine would be of the same order of magnitude as the total natural evaporation from the Rhine.
In the foggy winter months of the year, the increase in evaporation is at its maximum. The study thus comes to the conclusion that a sharp increase in fog formation in consequence of heating of the river cannot be excluded, at least in the proximity of the river.