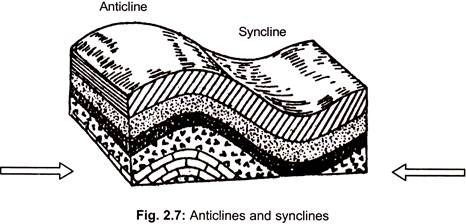
ADVERTISEMENTS:
The evolution of the earth’s atmospheric composition (including O2) involves significant interactions with biosphere, hydrosphere, and lithosphere. About 3.5 billion year ago, an interesting development occurred in the extensive waters of the primordial earth that profoundly affected the evolution of the atmosphere.
Today, the dry atmosphere consists primarily of diatomic nitrogen (N2) and diatomic oxygen (O2). Nitrogen is a highly stable gas that comprises 78 per cent of the present-day atmospheric volume. The abundance of N2 has increased as a percentage of the total atmospheric volume primarily because it is not removed as effectively from the atmosphere as are most other atmospheric gases. The residence time-the mean length of time that an individual molecule remains in the atmosphere of N2 is believed to be approximately 16.25 million years.
The next most abundant gas in the present-day dry atmosphere is oxygen, comprising approximately 21 per cent of the atmospheric volume today. Added to the quantity of nitrogen, these two gases constitute 99 per cent of the dry atmosphere. About 0.93 per cent of the remaining 1 per cent is composed mostly of argon (A), and a wide array of atmospheric trace gases constitute the remainder.
ADVERTISEMENTS:
Of these, CO2 is the fourth most abundant gas in the dry atmosphere, with 0.038 per cent of the dry atmosphere, or 380 parts per million (ppm). It plays an especially important role in maintaining the temperature of the planet at a level comfortable for life in its present form. The earth’s early atmosphere apparently contained far more CO2 than today’s, and little or no O2. So where did most of the CO2 go after outgassing in the primitive atmosphere, and how did O2 come to replace it?
Single-celled organisms called prokaryotes began to appear. These simple ancestors of bacteria and green algae absorbed nutrients directly from the surrounding environment. Prokaryotes allowed for the release of CO2 to the atmosphere as a byproduct of fermentation, the process by which simple organisms acquire energy through the breakdown of food.
The evolution of prokaryotes led to more complex multi-celled organisms called eukaryotes, which contain more complex internal structures, and release even more CO2 into the atmosphere. Most life on earth evolved from the further development of eukaryotes.
However, prokaryotes and eukaryotes would have had to develop in the oceans because without oxygen in the atmosphere, the protective ozone (O2) layer could not have formed to protect terrestrial life from the harmful ultraviolet (UV) radiation emitted by the Sun. Over time, CO2 continued to accumulate, as it became a larger and larger component of the atmospheric volume.
By about 3 billion years ago, another major development in the history of life on earth apparently caused another major change to the atmospheric composition. The early evolution of aquatic green plants led to a significant extraction of CO2 from the atmosphere as a requirement of photosynthesis – the process of deriving energy through the breakdown of food-and the sequestration of atmospheric CO2, from the biomass of those plants. Photosynthesis releases O2 into the atmosphere as a byproduct.
ADVERTISEMENTS:
Therefore, as green plants began to populate the earth, first in the oceans and later on land – after the presence of O2 gradually led to the formation of ozone (O2) and the O2 layer-atmospheric CO2 simultaneously increased. It is believed that at least some of the early prokaryotes and eukaryotes had used photosynthesis to break down their food, so some beginnings of the ozone layer probably predated the evolution of green plants.
Today most of the atmospheric CO2 is stored in vast quantities of sedimentary rock, originally extracted from the atmosphere by living things. The amount of atmospheric O2 present today represents a similar percentage to that of CO2 in the early atmosphere.
i. Constant Gases:
Nitrogen, oxygen and argon are called the “constant gases” because their concentration has remained virtually the same for much of recent earth history.
ADVERTISEMENTS:
Nitrogen (78%) is a relatively inert gas produced primarily by volcanic activity. It is an important component of protein in meat, milk, eggs and the tissues of plants, especially grains and members of the pea family. It cannot be ingested directly by organisms but made available to plants, and then to animals, by compounds in the soil. Most atmospheric nitrogen enters the soil by nitrogen-fixing microorganisms.
Oxygen (21%) is important for plant and animal respiratory processes. It is also important to chemical reactions (oxidation) that breakdown rock materials (chemical weathering). Without oxygen, things cannot burn either. Free oxygen in the atmosphere is a product of plant photosynthesis. Plants take up carbon dioxide and in the process of photosynthesis release oxygen.
Argon (.93%) is a colourless, odorless relatively inert gas, the reason it use to electric light bulbs, fluorescent tubes. It is used to form inert atmosphere for arc welding, and growing semiconductor crystals.
ii. Variable Gases:
The so called “variable gases” are those present in small and variable amounts. These include carbon dioxide, methane, ozone, water vapour, and particulates among others. Even though they represent a tiny portion of the atmosphere as a whole, they exert a great control over our environment.
iii. Carbon Dioxide:
Carbon dioxide (CO2) makes up only .036% of the atmosphere by volume. Carbon dioxide is essential to photosynthetic processes of plants. Huge quantities of carbon are stored in plant tissue, deposits of coal, peat, oil, and gas. Carbon dioxide is taken in by plants and during photosynthesis is combined with water and energy to form oxygen and carbohydrates. The stored carbohydrates are used to fuel plant respiration and growth.
Carbon is also stored in limestone rocks that have formed by the compaction of carbonate-rich shells of ocean life. Because vegetation takes in so much carbon dioxide, we often refer to plants as a “sink” for it.
Carbon dioxide in the atmosphere varies throughout the year, decreasing slightly during the summer as plants leaf out, and then increases during the winter as plants go dormant and photosynthesis decreases. The zig-zag pattern of carbon dioxide measurements taken at Mauna Loa, Hawaii in Figure 2.1 below illustrates this seasonality.
iv. Methane:
Methane (CH4) is a greenhouse gas contributing to about 18% of global warming and has been on the rise over the last several decades. Though methane makes up far less of the atmosphere (.0002%) than carbon dioxide, it is 20 times more potent than CO2 as a greenhouse gas. Methane is a product of the decomposition of organic matter, with major natural sources being that which occurs from wetlands, termites, the oceans, and hydrates.
A major source of methane is from termites. Termites eat wood and produce methane as a result of the breakdown of cellulose in their digestive tracts. They are thought to be responsible for 11% of the methane in the atmosphere (some estimates are as high as 20% – 40%). The clearing of the rain forests greatly impacts termite populations and in turn the methane content of the atmosphere. When a patch of rain forest is cleared, termite populations explode due to the ample food source that is left behind.
v. Ozone:
Ozone (O3) is both beneficial and harmful to life on Earth. Much of the ozone in the atmosphere is found in the stratosphere. Here, ozone absorbs UV light from the Sun preventing it from reaching the surface. Without this blanket, humans would be exposed to serious Sun burn and potential risk of skin cancer. Ozone is also found in the lowest layer of the atmosphere, the troposphere.
Here ozone can act as an eye and respiratory irritant. Ozone also causes cellular damage inside the leaves of plants causing brown splotches, impairing carbon dioxide uptake and disrupting the photosynthetic apparatus. Such damage can cause economic losses through reduced crop yields. It also damages the carbon ”sink” role of vegetation leaving more carbon dioxide in the atmosphere to enhance the greenhouse effect and potential global warming.
Human-produced compounds such as chlorofluorocarbons and halides containing chlorine and bromine destroy ozone, and have disrupted the fragile stratospheric ozone layer over Antarctica and the Arctic. Ozone depletion over Antarctica occurs during the spring when sunlight returns to the South Pole and the temperatures are still very cold.
vi. Water Vapour:
Water vapour is an extremely important gas found in the atmosphere. It can vary from 4% in the steamy tropics to nearly non-existent in the cold dry regions of the Antarctic. Water vapour is a good absorber of Earth’s outgoing radiation and thus is considered a greenhouse gas.
When water vapour is converted to a liquid during condensation, clouds are formed. Clouds are good absorbers of radiation given off by the Earth’s surface. The absorption of this energy raises the temperature of the air.
But clouds are generally light-coloured and hence reflect incoming solar radiation off their tops. The reflected light is sent back to space, never reaching the ground to warm the Earth. Thus clouds can have either a warming or a cooling effect on air temperature. It has been thought that these effects balance one another out but National Public Radio’s All Things Considered report suggests that this might not be true, forcing climatologists to rethink the issue.
vii. Particulates and Aerosols:
Atmospheric particulates and aerosols are very small particles of solid or liquid suspended in the air. Particulates and aerosols play several important roles in atmospheric processes. Particulate matter includes dust, dirt, soot, smoke, and tiny particles of pollutants. Major natural sources of particulates are volcanoes, fires, wind-blown soil and sand, sea salt, and pollen. Human sources such as factories, power plants, trash incinerators, motor vehicles, and construction activity also contribute particulates to the atmosphere.
Particulates are very effective at altering the energy and moisture balances of the Earth system. Particulates diffuse sunlight reducing the amount and intensity of solar radiation reaching the Earth’s surface. The most spectacular sunrises and sunsets are a result of light being refracted from particulates in the atmosphere. Particulates will also reflect sunlight back out to space, never letting it reach the surface.
Decreasing significant amounts of incoming solar radiation can cause global temperatures to decrease. The eruption of Mt. Pinatubo in 1991 caused a .5°C decrease in global temperatures. However, particulates can absorb long-wave radiation emitted by the Earth, causing the atmosphere to warm as well. Particulates serve as condensation nuclei for water.
In order for water to change from a gas to a liquid, a nucleus upon which water vapour can attach itself is nearly always required. Without particulates, little water would condense to form clouds and precipitation.
NASA scientists using satellite data and computer models found black soot from incomplete combustion may be contributing to changes in sea ice, snow and atmospheric temperatures near the North Pole. They found the timing and location of rising temperatures and loss of sea ice during the end of the 20th century is consistent with a significant rise in human produced aerosols.
Their models suggest that one third of the soot comes from South Asia, one third from biomass burning, and the rest from Russia, Europe, and North America. Soot deposited on snow and sea ice decreases the surface reflectivity causing more sunshine to be absorbed. Airborne soot warms the Arctic atmosphere and affects weather patterns and clouds.
Predicting Atmospheric Composition:
It is undeniable that the climate of Earth has seen numerous changes over time. Our current climate is undergoing changes in unprecedented ways for modern times. Measurements of atmospheric temperature show a clear upward trend while many regions are gripped in years-long drought. Most geoscientists concur that the current warming trend is related to human activities. Some disagree and suggest it is a natural fluctuation similar to that which the earth has experienced in the past.
To understand how climate has changed in the past, and more importantly, what will happen in the future, geoscientists must rely on models. Models are merely representations of the real world and are constrained by our understanding of earth processes. Scientist must make assumptions to fill in gaps where understanding or data is lacking. As a result, the output of a model inherently has a degree of uncertainty associated with it.
Predicting the changes in gaseous composition of the atmosphere is a difficult exercise. How the gaseous composition of the atmosphere will change in the future depends a great deal on the impact of human activities and our ability to control emissions. In addition to changes brought about by humans, increasing temperatures as a result of an enhanced greenhouse effect will impact the concentration of various gases in earth’s atmosphere.
Prediction versus Projection:
Uncertainty over how population will grow, economies develop, and technological advancement makes it difficult at best to predict the future composition of the atmosphere. Bodies like the Intergovernmental Panel on Climate Change (IPCC) make projections based on models. Though quite sophisticated, models of earth systems are constrained by our knowledge of environmental processes.
The unpredictability of human advancement and the state of our understanding of climate dynamics leaves uncertainty in these projections. Thus geoscientists use statistics and their expert judgment for factors that elude quantification to determine the likelihood for the outcome of their model projections.
Future concentrations will not necessarily be geographically defined due to the fluid nature of the atmosphere. The exception may be water vapour, due to the unequal distribution of available water to evaporate into the air. However, the sources of future contributions to the gaseous composition of the atmosphere are certainly geographically distributed, especially those from human activities. The race toward economic development will shift the largest contributors to the developing world.
IPCC Storylines and Scenarios:
The Intergovernmental Panel on Climate Change (IPCC) is recognized by many as the foremost authority on climate change. Their most recent analysis of climate change (2007) utilised four different “storylines” that represent the range of driving forces and emission behind climate change. Each storyline posed several different scenarios, grouped as “families” to examine the outcome of models that use similar assumptions about the driving forces, and some that do not.
Projected Increases in Greenhouse Gases:
Projected increases from the four story lines are shown in Figure 2.7. All but the A2 show an increase in CO2 followed by a decrease or leveling in CO2 emissions the coming century. The A2 scenario shows an increasing rate of emissions through time. In some sense, this is the “worst case scenario” with a continuously increasing population using more resources, slow technological change, and lack of global unity in tackling these issues.
At the opposite end of the spectrum is the ”Utopian” B1 scenario. The B1 scenario assumes that low fertility with low mortality and central migration rates, global population peaks mid-century, and then continually decreases toward 2100. Resource-efficient and renewable technology adoption diffuses rapidly through the world economy.
The greening of material production and energy use helps lower carbon dioxide emissions from industry and transportation. The A2 and B1 scenarios represent two ends of the spectrum of possible outcomes. But in both cases, the importance of global population change and attendant demand for resources has a strong impact on emissions of greenhouse gases and stress on the physical environment.
It may be just as likely for the changes in the composition of the atmosphere to lie somewhere between these extremes. The A1 market-driven scenarios of decreasing population growth coupled with rapid expansion of technology can yield reductions of greenhouse gases as well. The IPCC does not suggest the likelihood of any particular outcome. They should be seen for what they are, scenarios, what could happen given particular conditions. Thus they serve policy makers with a kind of roadmap to the future, however uncertain it might be.